Tenofovir-related
Kidney Toxicity Linked to High Drug Concentrations, May Not Always
Be Reversible
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
Three recently published studies shed further light
on kidney (renal) toxicity associated with tenofovir
(Viread, also in the Truvada
and Atripla
coformulations), which is approved for treatment of
both HIV and hepatitis
B. According to a research letter in the April
24, 2010 issue of AIDS, kidney function abnormalities
may result from higher than expected drug concentrations.
A related study found that kidney function may not return
to normal after discontinuing tenofovir, while a third
report indicated that kidney-related biomarker changes
were observed in a small number of children with prolonged
tenofovir use. |
|
 |
 |
 |
 |
 |
 |
 |
By
Liz Highleyman
The
issue of kidney toxicity caused by tenofovir remains controversial
due to conflicting study data. Kidney impairment was not observed
in pivotal trials that led to the drug's approval, but those studies
excluded people with pre-existing kidney damage. Several real-world
clinical studies have found an increased likelihood of kidney
toxicity in a small proportion (typically around 1%-2%) of people
taking tenofovir, especially those with other risk factors such
as older age and high blood pressure; other studies, however,
have not seen this association.
|
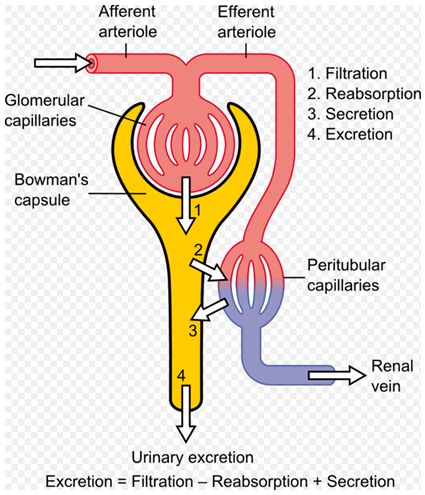
Diagram
showing the basic physiologic mechanisms of the kidney
(Source: Wikipedia)
|
|
 |
Tenofovir
Concentration
Sonia Rodriguez-Novoa from Hospital Carlos III in Madrid, Spain,
and colleagues looked at the relationship between tenofovir exposure
and kidney tubular dysfunction.
The kidneys perform 2 primary functions; the glomerulus filters
out various substances from the blood, while the tubules reabsorb
water. Tenofovir is processed and excreted in the kidneys by a
combination of glomerular filtration and active tubular secretion.
Tenofovir seldom affects glomerular function (as indicated by
glomerular filtration rate), the study authors noted as background,
but abnormalities in kidney tubular function appear to be more
common.
Variations or polymorphisms in genes encoding transporter proteins
involved in tenofovir elimination might help explain individual
differences in kidney toxicity, they added, since slowed elimination
might lead to drug overexposure in the kidneys.
The researchers first conducted a larger study of kidney tubule
function, determined by 24-hour urine monitoring, among 284 HIV
patients; 154 were on tenofovir-containing antiretroviral therapy
(ART), 49 were on ART without tenofovir, and 81 were treatment-naive.
They found that significantly more participants taking tenofovir
exhibited kidney tubular dysfunction compared with the other 2
groups (22%, 6%, and 12%, respectively; P < 0.05).
In the present sub-study, the investigators prospectively analyzed
92 tenofovir recipients. Kidney tubular dysfunction was determined
on the basis of glucose, amino acids/protein, and beta-2-microglobulin
in the urine, altered reabsorption of phosphorus, and abnormal
uric acid excretion (any 2 manifestations). The median duration
of tenofovir exposure was 33 months.
Results
 |
19%
of participants met criteria for kidney tubular dysfunction,
while 81% had normal tubular function. |
 |
The
2 groups were well-matched with regard to sex, age, weight,
high blood pressure, hepatitis C coinfection, length of tenofovir
exposure, and use of kidney-toxic drugs; however, those with
kidney dysfunction were more likely to have diabetes. |
 |
Creatinine
clearance (an indicator of glomerular function) was normal
and comparable between the 2 groups. |
 |
The
median tenofovir plasma trough (lowest between doses) concentration
was significantly higher in patients with kidney tubular dysfunction
compared to those with normal function (182 vs 106 ng/mL,
respectively; P = 0.001). |
 |
A
tenofovir plasma concentration of 160 ng/mL was the best threshold
for kidney tubular dysfunction risk, with 61% sensitivity
and 80% specificity. |
 |
In
a multivariate analysis including all relevant risk factors,
only tenofovir plasma levels > 160 ng/mL were associated
with kidney tubular dysfunction (odds ratio [OR] 4.8, or nearly
5-fold higher risk). |
 |
Multivariate
analysis also showed that female sex (OR 71) and the ratio
of body weight to plasma creatinine (OR -1.19) were independently
associated with higher tenofovir plasma levels. |
"This dose-dependent effect further supports an involvement
of tenofovir in tubular dysfunction," the researchers concluded.
"If tubular damage persists for long periods in the absence
of renal insufficiency, laboratory and clinical manifestations
other than those associated with kidney failure may develop ([e.g.,]
premature osteoporosis due to bone mineral loss) under long-term
tenofovir therapy," they elaborated.
"The mechanism of kidney tubular damage by tenofovir is probably
related [to] its renal clearance, supporting that higher tenofovir
plasma levels may lead directly to a greater accumulation of tenofovir
in the renal tubular cells and, consequently, to kidney toxicity,"
they continued.
This concentration-dependent effect is important, they suggested,
because tenofovir dose reduction might be an option, especially
for people with chronic hepatitis B.
Incomplete
Reversibility
In
the second study, published in the February
19, 2010 advance online edition of the Journal of Acquired
Immune Deficiency Syndromes, Karen Wever and Andrew Carr
from St. Vincent's Hospital in Sydney and colleagues assessed
the reversibility of tenofovir-related kidney toxicity.
Some
studies have shown that kidney toxicity resolves fairly quickly
after stopping tenofovir, but this has often been based on short-term
follow-up or looked only at creatinine clearance, a less sensitive
measure of kidney function.
This
study included 24 HIV positive men who discontinued tenofovir
due to kidney function impairment, defined as an estimated glomerular
filtration rate (eGFR) below 90 using the Modified Diet in Renal
Disease (MDRD) equation; lower numbers (in mL/min/1.73 m) indicate
poorer glomerular function, and 60 is the usual threshold for
chronic kidney disease.
The
median duration of tenofovir use was 30 months. The investigators
retrospectively analyzed changes in eGFR during a median follow-up
period of 13 months after stopping tenofovir.
Results
 |
Most
participants had some degree of kidney impairment before they
started tenofovir, with a median eGFR of 74. |
 |
At
the time of tenofovir discontinuation, the median eGFR had
fallen to 51. |
 |
Median
eGRF increased to 58 during follow-up, but did not return
to the pre-tenofovir level. |
 |
Only
10 patients (42%) returned to their pre-tenofovir eGFR. |
 |
Results
were similar using an alternative estimation method, the Cockcroft-Gault
equation. |
 |
Results
were also similar after excluding patients who had shorter
follow-up after stopping tenofovir. |
"In this population, tenofovir-related renal toxicity was
not always fully reversible," the study authors concluded.
"Greater
eGFR improvement was significantly associated with more rapid
decline in eGFR on tenofovir therapy," while a slower decrease
over time predicted persistent impairment, they noted. Improvement
was also more likely among people who took tenofovir with a protease
inhibitor (as opposed to a NNRTI), with a trend for shorter duration
of tenofovir exposure.
Based
on these findings, the researchers suggested that a decline in
eGFR -- even if gradual and not falling below 60 -- "may
merit discontinuation of tenofovir to avoid permanent renal dysfunction."
Kidney
Function in Children
Few
studies have looked at kidney function impairment in HIV positive
children.
As
reported in the February
20, 2010 issue of AIDS, Ali Judd from the Medical Research
Council Clinical Trials Unit in London and colleagues investigated
the link between tenofovir use and abnormal kidney function in
a large cohort of children on ART.
This nested case-control study included 456 ART-exposed children
(age 2-18) in the Collaborative HIV Paediatric Study, which represents
about 95% of HIV positive children in the UK and Ireland.
The researchers analyzed serum (but not urine) biochemistry data
collected during 2002-2008 at 7 hospitals. Case patients had either
confirmed hypophosphatemia (elevated phosphate) or an eGFR less
than 60. Each case patient was matched with 3 control children
seen at the same hospital.
Results
 |
20
of 456 children (4.4%) had hypophosphatemia and 1 had eGFR
less than 60. |
 |
10
of 20 case patients (50%) had taken tenofovir-containing ART
for a median of 18 months as part of second-line or salvage
therapy, compared with 11 of 60 control subjects (18%). |
 |
The
incidence of hypophosphatemia was 4.3 per 100 person-years
among tenofovir recipients, compared with 0.9 per 100 person-years
among those not exposed to tenofovir. |
 |
In
a multivariable analysis, only tenofovir exposure during the
previous 6 months was associated with hypophosphatemia (odds
ratio 4.81; P = 0.01). |
 |
Among
6 of 10 children with hypophosphatemia who had at least 4
subsequent measurements, phosphate values returned to normal
after tenofovir was discontinued. |
 |
Among
4 children with 3 or fewer subsequent measurements, phosphate
values rose but remained below normal. |
"Hypophosphatemia was uncommon (4%), but was associated with
prolonged tenofovir use, and was generally reversible following
tenofovir withdrawal," the study authors concluded.
These findings "highlight the importance of continuing to
monitor longer-term renal function, in particular tubular function,
especially in those taking tenofovir," they added. "Further
studies assessing urine biochemistry measures, which more accurately
indicate renal tubular damage, are required."
Investigator affiliations:
Rodriguez-Novoa
study: Department of Infectious Diseases, Hospital Carlos III,
Madrid, Spain; Service of Infectious Diseases, University of Torino,
Torino, Italy; Department of Nephrology, Hospital Infanta Leonor,
Madrid, Spain.
Wever study: HIV, Immunology, and Infectious Disease Unit and
Centre for Applied Medical Research, St. Vincent's Hospital, Sydney,
Australia; VU Medical Centre, Amsterdam, Netherlands.
Judd study: Medical Research Council Clinical Trials Unit, London,
UK.
5/18/10
References
S Rodriguez-Novoa, P Labarga, A D'avolio, and others. Impairment
in kidney tubular function in patients receiving tenofovir is
associated with higher tenofovir plasma concentrations. AIDS
24(7): 1064-1066 (Abstract).
April 24, 2010.
K
Wever, MA van Agtmael, and A Carr A. Incomplete reversibility
of tenofovir-related renal toxicity in HIV-infected men. Journal
of Acquired Immune Deficiency Syndromes (Abstract).
February 19, 2010 (Epub ahead of print).
A Judd, KL Boyd, W Stöhr, and others. Effect of tenofovir
disoproxil fumarate on risk of renal abnormality in HIV-1-infected
children on antiretroviral therapy: a nested case-control study.
AIDS 24(4): 525-534 (Abstract).
February 20, 2010.
